Course Project
For the course project, you will be studying thermo-chemical ammonia synthesis on metallic clusters and surfaces. Each student will be assigned one metal or bimetallic alloy. The due date is 3/13 at 11:59 PM (hard deadline).
Turn in your final report by emailing a PDF file to all TA’s:
ctsai89@stanford.edu, aayush@stanford.edu, shaama@stanford.edu, ambarish@stanford.edu
Introduction
The thermo-chemical synthesis of ammonia is accomplished through the Haber-Bosch process, where nitrogen gas reacts with hydrogen gas via:
This process is crucial for the industrial production of fertilizers and chemical feedstocks. Typically, an iron catalyst is used to stabilize the bond-breaking of the N2 species. The reaction can be separated into elementary reaction steps (Honkala et. al. (2005) for more details):
A free energy diagram is illustrated below:
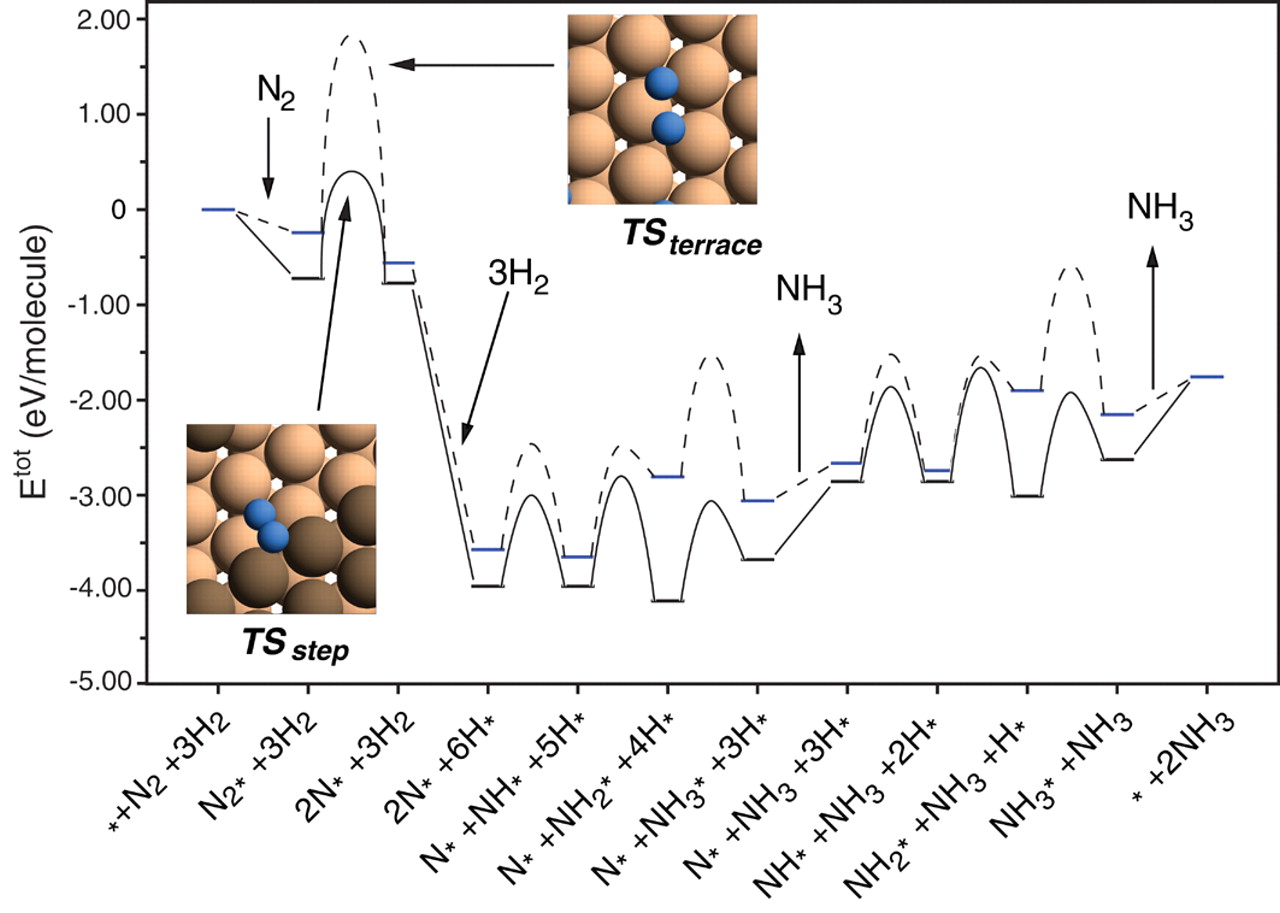
Ammonia synthesis pathway on a Ru catalyst (Honkala et. al. (2005))
Due to the high operating pressures and temperatures required for this reaction, alternative catalysts are still needed for this process. Medford et. al. (2015) have suggested that the linear scaling between the dissociation energy of N2 and its transition state energy prevents most catalysts from achieving a high rate. Assuming that the bond-breaking of N2 is rate limiting, then traditional metal catalysts have a transition state that is too high in energy. This is illustrated in the filled contour plot below, where the turnover frequency is plotted as a function of the transition state energy of the first N2 bond breaking (EN-N) and the dissociation energy (∆Ediss). A catalyst would need to behave differently from these extended surfaces in order to land in a more active region of the map.
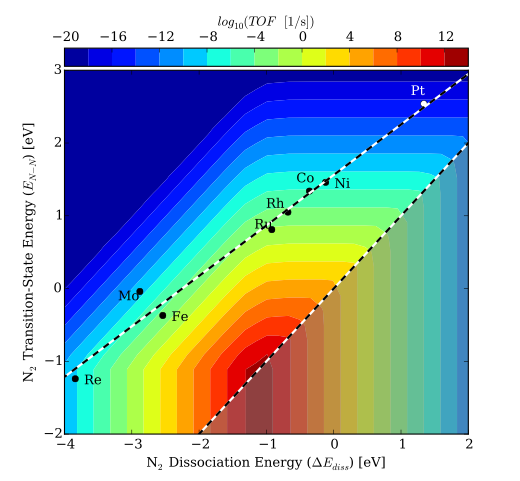
Filled contour plot for the turnover frequencies (Singh et. al. (2016))
We will be exploring 13-atom metal clusters as a system where such configurations might be found.
Your goals for the project will be to: (1) explore this reaction to find unique adsorption configurations where the dissociation energy leads to a more favorable transition state energy, and (2) explore additional relations between the reaction intermediates in the pathway. You will be studying 13-atom metal clusters (M13) consisting of either pure metals or binary alloys.
Calculations
For Sherlock, create a CHE444Project folder in your $SCRATCH directory by running:
mkdir $SCRATCH/CHE444Project/
for CEES:
mkdir /data/cees/$USER/CHE444Project
You may run the exercises in any directory (as long as it is under $SCRATCH for Sherlock and /data/cees/$USER for CEES), but keep all the final files for the project organized.
For the first step, N2 → 2N*, you are required to calculate the transition state. To do this, you will need to calculate the final adsorbed state with two nitrogen atoms (2N*).
To describe the full reaction on your catalytic system, you will need to calculate the adsorption energies of all intermediates, in their most stable configuration (N*, NH*, NH2*, NH3*, H*). A mean field approximation can be used in the analysis (e.g. ∆E2NH = 2∆ENH). You are not required to calculate the transition states for these steps, though you are welcome to.
In summary:
- Structural relaxations on both your assigned M13 cluster and a (111) surface for the same metals. Project Part 1
- For the M13 cluster only: Adsorption energies for the remaining intermediates in the adsorbed state (N*, NH*, NH2*, NH3*, H*). Check all possible sites in order to determine optimal adsorption configurations. Project Part 2
- For both M13 cluster and metal surface: Fixed bond length (FBL) calculation for the activation barrier for N2 → 2N*. You will need to perform an adsorption energy calculation for 2N*, which will serve as the starting point for the FBL. Project Part 3
- Vibrational analysis for the transition state and the adsorbed states. Calculation of the reaction rate and also a free energy diagram with some temperature and pressure dependence. Project Part 3
Once you have finished the required calculations, you are free to explore other features of the reaction as you see fit.
IMPORTANT:
When you have finished all your calculations. Confirm that your results are organized in the following way:
../CHE444Project/Adsorption/
../CHE444Project/Adsorption/Surface/
../CHE444Project/Adsorption/Surface/2N/
../CHE444Project/Adsorption/Surface/2N/config1
...
../CHE444Project/Adsorption/Cluster/
../CHE444Project/Adsorption/Cluster/2N/
../CHE444Project/Adsorption/Cluster/NH/
../CHE444Project/Adsorption/Cluster/NH/config1
../CHE444Project/Adsorption/Cluster/NH/config2
...
../CHE444Project/TransitionStates/Cluster/2N_to_N2/
../CHE444Project/TransitionStates/Cluster/2N_to_N2/config1
...
../CHE444Project/Vibrations/N/
../CHE444Project/Vibrations/2N/
...
where CHEMENG444Project is your project directory. You should rename config1 to something that describes the binding configuration, such as BrBr for two bridging sites. You should have one calculation per directory. Run the following to copy all your files into the shared course directory, so your classmates may access the results.
On Sherlock, from your project directory, run:
/scratch/PI/suncat/chemeng444_2016/submit
On CEES, from your project directory, run:
/data/cees/cheme444/submit
Analysis
Your analysis should include the following:
- Discussion of the optimal binding configurations on the surface
- Comparison between the (111) surface and the M13 cluster
- Analysis of rate as a function of the temperature
Beyond these points, you may discuss anything you find interesting. Here are some ideas:
- Do the reaction intermediates (adsorbates) show the same dependence on surface site or surface termination?
- What are other factors that can affect adsorbate-metal interaction?
- How important is the coordination number of the metal? Compare the metal cluster to the metal surface.
You are welcome to share data amongst your peers to discuss broader trends. (We encourage you to use Piazza for questions and discussions so you can help each other troubleshoot and share data. You may also include additional calculations into your project (scripts available), but this is not required:
- Density of states calculations on the surfaces
- Error analysis using the BEEF ensembles
If you need the energy of the fixed clusters, they are available here.
Report
Your report should be between 3 to 5 pages long including figures and tables. Please be succinct and organize it in the following way:
- Introduction (brief) - don’t write too much
- Calculation details
- Results and discussion
- Conclusion (brief)
Research Paper
We expect that your results will form the basis of a manuscript that we will be putting together as a class. For previous class papers:
- (2011) Finite-Size Effects in O and CO Adsorption for the Late Transition Metals, Topics in Catalysis
- (2015) Direct Water Decomposition on Transition Metal Surfaces: Structural Dependence and Catalytic Screening, Catalysis Letters.
Notice how much the class size has grown!
Grading
- 30% exercises
- 20% write-up
- 20% kinetics
- 30% calculations
Summary of Requirements
At a minimum you should accomplish the following:
- Complete the three exercises.
- Setup a M13 cluster and a (111) surface and calculate adsorption energies for all intermediates.
- Calculate transition states for the first step N2 dissociation) using the fixed bond-length method. Extra credit for calculating the hydrogenation barriers.
- Vibrational frequency and free energy calculations (initial, transition, and final states, and all adsorbed intermediates).
- Analysis
- Optimal adsorption sites (relation to transition states)
- Kinetic rate analysis
- Report (3~5 pages maximum)
Email your final report as a PDF document to:
ctsai89@stanford.edu, aayush@stanford.edu, shaama@stanford.edu, ambarish@stanford.edu